Genome editing:
A real promise for future agriculture
Led by genome editing, new breeding techniques (NBTs) are an evolving spectrum of technologies targeting changes in plant genetic material. Owing to their precision, speed, low cost, and simple application, genome editing can introduce valuable traits to plants and enhance yield or productivity growth. We have already seen NBT products reach consumers, the first being Calyno oil, a soybean cooking oil that claims to have higher oleic acid than its competitor.
The regulatory dispute
The legal clarity of emerging technologies and derived products is an ongoing international discussion as some mechanisms of NBTs overlap with genetic modification (GM) (1-3). It should be born in mind that some outcomes of NBTs are considered GM as they include foreign genes, other outcomes are indistinguishable from conventional counterparts (non-GM). The current regulatory landscape of NBTs including genome editing is quite diverse as shown in the world map of Figure 1. In the United States (US), most South American countries (e.g. Argentina, Brazil), Australia and Japan, genome-edited products are regulated on the final product. Product-based regulation of genome-edited products makes them exempt from genetically-modified organisms (GMOs) regulation as long as they are transgenic-free (i.e. final product does not contain foreign genetic material from non-compatible species).
The situation is different in the European Union (EU). As of July 2018, the Court of Justice of the EU (CJEU) ruled organisms obtained by mutagenesis are GMOs within the meaning of the GMO Directive (Case C-528/16). The distinctiveness of the global status of genome editing originates from the tensions between process- (i.e. a system that differentiates regulatory requirements based on the process used to make the product) and product-focused (i.e. approach bases regulatory requirements on the characteristics of the final product, regardless of the process by which it was made) regulatory frameworks. While the EU adopts process-based regulations, Canada, the US and Argentina, along with others implement a product-based approach (4).

Source:(5)
Tackling the challenge
To better understand the regulatory considerations surrounding emerging biotechnologies, we polled international plant biotechnology experts on what approach nations should agree upon to accommodate NBTs and their derived products. In late 2018 into January 2019, an online survey was emailed to a panel of 479 international scientists, government officials, and agribusiness professionals with related backgrounds and experiences in biotechnology. The panel of experts has been surveyed on several topics surrounding novel biotechnologies including their expert views on how to regulate NBTs and their resulting products (6). This blog summarizes the key results discovered from the panel responses and recent publication in Biotechnology Reports.
Expert opinion on the CJEU judgment of directed mutagenesis
When asked whether experts support or oppose the 2018 CJEU ruling, we found that 75% of surveyed experts were not in favour of the CJEU judgment. The rationale behind such criticism for the ruling is that many participants were concerned about the rigidity of EU policy governing biotechnology, namely a process-based system guided by the precautionary principle (PP). Also, several respondents believed that the ruling was politically driven and ignored scientific evidence(6).
Several experts predict that the ruling will potentially limit innovative research that will result in EU farmers having reduced access to new crop varieties. Others worried “it will amplify the global food gap, hurt sustainability, and disrupt international trade” (6). Potential issues arising from the EU regulatory system include limiting small farmers’ access to affordable technology with low-environmental impacts, increased difficulty for small businesses to develop new varieties and a range of impacts inside and outside the EU (e.g. 4, 7-9). According to Purnhagen et al. (10): “[i]f anything, the CJEU’s judgment underscores is the need for regulatory reform in the EU”.
How should we regulate genome-edited products?
Public confusion about food safety and health risks (38%), the cost of regulatory approval and biosafety compliance (34%) and trade (32%) were identified as key challenges participants have related to the ability to use NBTs to develop new crops (6). Since the emergence of NBTs, regulatory uncertainty has been a burden to many broadly applicable technologies (3).
Regardless of where experts live, most respondents (Table 1) chose product-based regulation—either stand-alone (59%) or combined with some process-based (i.e. hybrid) system (26%)—as ‘ideal’ approaches nations should adopt to regulate any risks related to transgenic outcomes of NBTs including genome editing (6).
Table 1: Expert opinion on how NBTs should be regulated by region (% of responses)
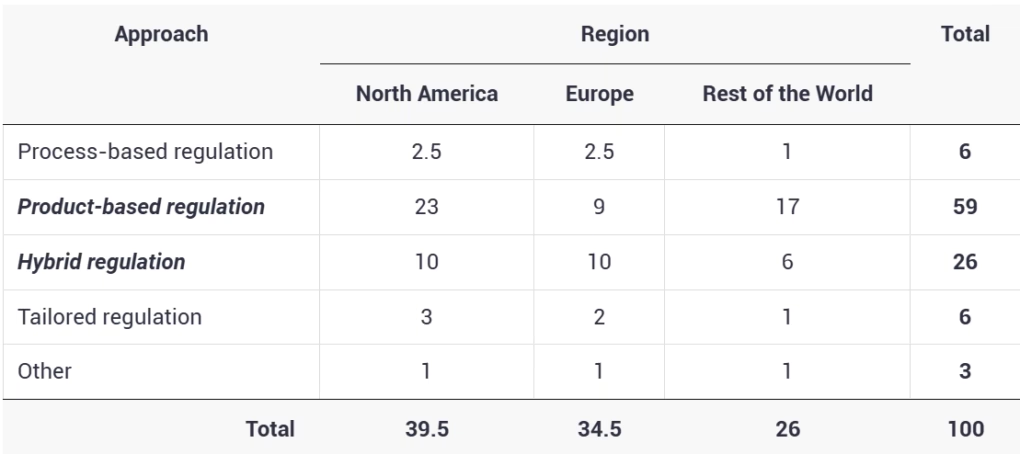
For many participants, the outcome of technology (either crop type or end-product) matters the most for farmers and consumers, as it’s the use that determines the risk or value of a new trait. Experts generally agree that the final product—regardless of how it’s developed—can generate potential risks. Experts further believe that a novel trait should be comprehensively characterized by its social impact, ethical considerations and sustainability (6, 11). As a process-based system may be unable to keep pace with emerging technologies, a product-based safety assessment is viewed as the “only scientifically valid approach”. One respondent stated that: “[f]ocusing on the process, in transgenesis, has led to bad decision-making, especially in Europe, and groundless fears in parts of the general public.” According to one expert, a product-based approach would help overcome the problem of defining a GMO, debates around novel crops, and thus consumer acceptance and understanding of the technology.
Regulation by a process is an unpopular choice
Survey results show only 6% of those surveyed viewed pure process-based systems as appropriate. The process-based regulatory systems may have been adequate at the end of the 20th century when other methods of characterization were lacking. But knowledge has advanced. There are now over 4,300 regulatory risk assessment decisions from 70 countries that have approved GM crops for food and feed use, none identifying risks that differ from conventional crop breeding (12). Our experts agreed that process-based regulatory systems are unsustainable.
A range of alternatives is supported including tailored regulation (i.e. case-by-case) that was recommended by 6% of the respondents. All early regulatory applications for products of genome editing (e.g. using CRISPR/Cas9) have been reviewed case-by-case in countries like Canada and the US, with some codification of the rules since then. Supporters of the case-by-case assessment argue that as applications of NBTs, including genome editing, vary so does the resulting trait or product and its potential impact(s). In our recent publication of Biotechnology Reports, we further explore what many of these alternative recommendations made by experts are, including regulation by trait, environmental factors and even a dual/hybrid system. One view is that process-assessment may be best suited to environmental concerns while product-based review can most effectively address food safety concerns. In other words, the systems can complement each other; embracing one of them might not be the best plan in the long run (6).
Conclusion
Food security and environmental sustainability might both be improved by the appropriate use of new biotechnology. Yet, its application mostly depends on how countries will de/regulate NBTs and their derived products. The complexity of the genome editing toolbox along with the possibility to use several techniques in combination increase the complexity to draw a comprehensive, one-size-fits-all regulatory approach (6). “There is some evidence that R&D funding is shifting from jurisdictions with process-based regulatory systems, such as that of the EU, to countries that adopt a product-based system, such as that used in most of the Americas. This would simply amplify the challenges of asynchronous use of crops derived from different technologies” (6).
Surveyed experts underline the need for the regulatory processes to modernise to address the challenges resulting from new technical opportunities. “Experts think the key to realizing genome editing’s potential is in regulatory transparency and open dialogue but the notion that an open dialogue with society on genome editing will lead to greater understanding needs to be validated, as recent structured dialogues have not led to greater acceptance or use.”
- Seyran E, Craig W. New Breeding Techniques and Their Possible Regulation. AgBioForum. 2018;21(1):1.
- Gatica-Arias A. The regulatory current status of plant breeding technologies in some Latin American and the Caribbean countries. Plant Cell, Tissue and Organ Culture (PCTOC). 2020;141(2):229-42. doi: 10.1007/s11240-020-01799-1.
- Lassoued R, Smyth SJ, Phillips PWB, Hesseln H. Regulatory Uncertainty Around New Breeding Techniques. Frontiers in Plant Science. 2018;9(1291). doi: https://doi.org/10.3389/fpls.2018.01291.
- Eriksson D, Kershen D, Nepomuceno A, Pogson BJ, Prieto H, Purnhagen K, et al. A comparison of the EU regulatory approach to directed mutagenesis with that of other jurisdictions, consequences for international trade and potential steps forward. New Phytologist. 2019;222(4):1673-84. doi: 10.1111/nph.15627.
- Metje-Sprink J, Sprink T, Hartung F. Genome-edited plants in the field. Current Opinion in Biotechnology. 2020;61:1-6. doi: https://doi.org/10.1016/j.copbio.2019.08.007.
- Lassoued R, Macall DM, Smyth SJ, Phillips PWB, Hesseln H. How should we regulate products of new breeding techniques? Opinion of surveyed experts in plant biotechnology. Biotechnology Reports. 2020:e00460. doi: https://doi.org/10.1016/j.btre.2020.e00460.
- Smyth SJ, Lassoued R. Agriculture R&D Implications of the CJEU’s Gene-Specific Mutagenesis Ruling. Trends Biotechnol. 2018. Epub 2018/10/09. doi: 10.1016/j.tibtech.2018.09.004. PubMed PMID: 30293646.
- Urnov FD, Ronald PC, Carroll D. A call for science-based review of the European court’s decision on gene-edited crops. Nature Biotechnology. 2018;36:800. doi: 10.1038/nbt.4252 https://www.nature.com/articles/nbt.4252#supplementary-information.
- Rodolphe B. CRISPR Craziness: A Response to the EU Court Ruling. The CRISPR Journal. 2018;1(4):251-2. doi: 10.1089/crispr.2018.29025.edi. PubMed PMID: 31021214.
- Purnhagen KP, Kok E, Kleter G, Schebesta H, Visser RGF, Wesseler J. EU court casts new plant breeding techniques into regulatory limbo. Nature Biotechnology. 2018;36:799. doi: 10.1038/nbt.4251.
- Lassoued R, Macall DM, Smyth SJ, Phillips PWB, Hesseln H. Risk and safety considerations of genome edited crops: Expert opinion. Current Research in Biotechnology. 2019;1:11-21. doi: https://doi.org/10.1016/j.crbiot.2019.08.001.
- Global Status of Commercialized Biotech/GM Crops in 2018: Biotech Crops Continue to Help Meet the Challenges of Increased Population and Climate Change [Internet]. http://www.isaaa.org/resources/publications/briefs/54/executivesummary/default.asp; 2018. ISAAA Brief 54-2018: Executive Summary
If you would like to know more about Dr. Lassoued’s research related to this posting you can find them on the publications page of the multi-year project Regulation of New Breeding Techniques webpage.




Pingback: Outlook into the future regulatory landscape for genome editing – SAIFood | plantlawyer